In 1790, the Italian physiologist Luigi Galvani noticed that the leg of a dissected frog twitches if it is simultaneously touched with two instruments made of different metals. At that time it was already known that muscles could contract under the influence of electric current, so Galvani correctly attributed this phenomenon to the action of electric current. True, he believed that electric current appears due to some physiological processes in the frog’s leg.
Another Italian scientist Alessandro Volta was able to give the correct explanation for this phenomenon. He found that this phenomenon was associated with the presence of two dissimilar metals in contact with the electrolyte, which was the blood of the frog, and the paw itself played only the role of a sensitive indicator of electric current [1]. Based on his studies of Volta in 1799. created the first chemical current source. In this device, Volta used copper and zinc electrodes immersed in a sulfuric acid solution.
Zinc reacts violently with acids. It is not zinc atoms that pass into the solution, but positive ions, so that an excess of electrons remains in the electrode, therefore, the zinc plate is negatively charged. In general, most metals become negatively charged when immersed in an electrolyte; a similar process occurs on the surface of a copper plate. But the excess of negative charges on the copper electrode is much less, which means that its potential is higher relative to the zinc electrode. If you connect copper and zinc plates with an external conductor, then electrons will begin to move from the zinc plate to the copper one, i.e. electric current will flow in the circuit [2].
The electrical voltage that occurs between the electrodes depends on what metals the electrodes are made of and on their interaction with the electrolyte. The voltage supplied by the element does not depend in any way on the area of the plates.
Often the voltage provided by one galvanic cell is not enough. Then they can be connected in series into batteries.
In general, making a chemical current source is not at all difficult: you need to place two plates of different metals in an electrolyte [3]. Such galvanic cells arise spontaneously. For example, if rain wets a roof covered with galvanized iron, there are probably scratches on the iron, so that both iron and zinc come into contact with water, which acts as an electrolyte. The zinc in such a pair will begin to actively degrade, but the iron will not be affected until all the zinc is destroyed. This is why iron is coated with a layer of zinc.
For the same reason, twisting copper and aluminum wires together is, to put it mildly, not the best idea. Galvanic corrosion will begin at the point of contact, which will lead to an increase in the electrical resistance of the contact, which in turn will lead to greater heat generation and even faster corrosion. All together this can cause destruction of the connection and even a fire.
Galvanic corrosion can be most clearly observed by the example of contacts of iron with zinc and copper in a salt solution. Iron clips were placed on zinc and copper plates and immersed in a salt solution.
A day later, the paperclip connected to the copper plate became covered with rust. While the paper clip, which was in contact with zinc, was not damaged at all.
Scientists have compiled an electrochemical series of metal voltages. The farther apart the metals are in this row, the higher the voltage produced by the galvanic cell composed of these metals. Thus, a gold-lithium pair can theoretically produce an electromotive force (EMF) of 4.72 V. But such a pair will not be able to work in an aqueous environment - lithium is an alkali metal that easily reacts with water, and gold is too expensive for such an application.
In practice, the Volta element has a number of serious disadvantages.
- Firstly, its electrolyte is a very caustic liquid - a solution of sulfuric acid. Liquid electrolyte is always inconvenient or even dangerous. It may splash or spill if the housing is damaged.
- Secondly, hydrogen will be released at the copper electrode of such an element. This phenomenon is called polarization. In many properties, hydrogen is very close to metals, so its bubbles will create an additional emf of polarization, tending to cause a current in the opposite direction [2]. In addition, gas bubbles do not allow electric current to pass through, which also leads to a weakening of the current. Therefore, you have to periodically shake the vessel, removing bubbles mechanically, or introducing special depolarizers into the electrolyte.
- Thirdly, during the operation of the Volta galvanic cell, the zinc electrode gradually dissolves. Theoretically, when the galvanic cell is not used, the destruction of the zinc electrode should stop, but since zinc almost always contains impurities of other metals, when they come into contact with the electrolyte, they play the role of a second electrode, forming a short-circuited element, which leads to galvanic corrosion of the zinc electrode [2] . In order to eliminate this drawback, it is necessary to use ultra-pure zinc or design to provide for the possibility of removing the zinc electrode from the electrolyte. So when the battery is not in use, the electrolyte should be drained from it.
But for demonstration purposes, all these disadvantages can be neglected if the sulfuric acid is replaced with a safer electrolyte.
Source of currents
There are two types of electrochemical cells: galvanic and electrolytic. The galvanic cell uses the energy released during a spontaneous redox reaction to generate electricity.
An electrolytic cell draws energy from an external source, using it to trigger an unexpected redox reaction.
Two cell types
The galvanic cell, the history of which officially began in the 18th century, gave rise to the development of the science of electrical engineering. While experimenting with electricity in 1749, Benjamin Franklin first coined the term "battery" to describe connected capacitors. However, his device did not become the first cell. The discoveries of the “Baghdad Battery” by archaeologists in 1936 are over 2,000 years old, although their exact purpose is still controversial.
Luigi Galvani, after whom the voltaic cell is named, first described “animal electricity” in 1780 when he passed current through a frog. He didn't know it at the time, but his device worked like a battery. His contemporary Alessandro Volta, after whom the “voltaic cell” is named, was convinced that “animal electricity” came not from the frog, but from something else, he worked hard on this and in 1800 invented the first real battery - the “voltaic heap” .
Alexandro Volt
In 1836, John Frederick Daniel, researching ways to overcome the problems of the voltaic heap, created his cell. This discovery was followed by the creation of the William Robert Grove cell in 1844. The first rechargeable battery was made from a lead-acid cell in 1859 by Gaston Plante, followed by the Callot gravity cell in 1860 and the Leclanche cell by Georges Leclanche in 1866.
Up to this point, all batteries were of the wet type. In 1887, Karl Gassner created the first dry cell battery, made from a carbon-zinc battery. The nickel-cadmium battery was introduced in 1899 by Waldmar Jungner along with the nickel-iron battery. However, Jungner was unable to patent it and in 1903, inventor Thomas Edison patented his slightly modified design.
Russian physicist Vasily Petrov in 1802 built the largest galvanic battery in the world, producing a voltage of 1500V. The construction required about 4200 cylinders made of copper and zinc with a diameter of 35.0 mm and a thickness of 2.5 mm. The battery was housed in a mahogany box treated with several layers of different resins. Petrov's experiments marked the beginning of modern electrometallurgy in arc furnaces.
Note! A major breakthrough in the galvanic direction of current sources occurred in 1955, when Lewis Urry, an employee of , introduced the general alkaline battery. The 1970s led to the nickel-hydrogen battery and the 1980s to the nickel-metal hydride battery
Lithium batteries were first created back in 1912, but the most successful type, the lithium-ion polymer battery used in most portable electronic devices today, was not released until 1996.
Method two: a jar of electrolyte
To assemble a device with your own hands, similar in design to the world's first battery, you will need a glass jar or glass. For the electrode material we use zinc or aluminum (anode) and copper (cathode). To increase the efficiency of the element, their area should be as large as possible. It would be better to solder the wires, but the wire will have to be attached to the aluminum electrode with a rivet or bolted connection, since it is difficult to solder.
The electrodes are immersed inside the can so that they do not touch each other, and their ends are above the level of the can. It is better to secure them by installing a spacer or a cover with slots. For the electrolyte we use an aqueous solution of ammonia (50 g per 100 ml of water). An ammonia aqueous solution (ammonia) is not the ammonia used for our experiment. Ammonia (ammonium chloride) is an odorless white powder used in soldering as a flux or as a fertilizer.
https://youtube.com/watch?v=yeWnPoQ3cSY
The second option for preparing the electrolyte is to make a 20% sulfuric acid solution. In this case, you need to pour the acid into the water, and in no case vice versa. Otherwise, the water will instantly boil and its splashes, along with the acid, will get on your clothes, face and eyes.
All that remains is to pour the resulting solution into the jar so that there is at least 2 mm of free space left to the edges of the vessel. Then, using a tester, select the required number of cans.
A self-assembled battery is similar in composition to a salt battery, as it contains ammonium chloride and zinc.
DIY galvanoplasty
It is not difficult to assemble an installation for electroplating at home; equipment and materials for electrochemical deposition of copper are freely available. The exception is sulfuric acid, the acquisition and use of which is possible only by an organization with a special permit.
There are ready-made galvanizing kits on sale, but their purchase will not always be justified - it is much cheaper to assemble the installation yourself using available equipment.
With the help of a galvanic installation, which we will discuss in this article, you will be able to obtain copies of artistic products, regardless of the material from which they are made, and also, having the skills of modeling from plasticine or clay, reproduce your own works in metal.
In addition, using the electroplating method, you can implement many interesting projects, for example, metallize woven or knitted lace to make openwork compositions, make metal herbariums from flowers and leaves, metallize fruits, finish glass or porcelain products, increasing the layer of copper according to previously specified pattern, and much more.
Electroplating can be an excellent choice not only as an interesting hobby, but with the right approach and perseverance, it can become the foundation for a future business.
Equipment for electroforming at home
Galvanic deposition of copper at home is carried out in containers of any geometric shape. The size of the galvanic container depends on the size of future products or reproduced compositions. The material can be different; containers made of glass, ceramics or plastic are suitable.
The second key element of a galvanic installation is the direct current source. To carry out the work, use a low voltage current in the range of 3-6 V. You can use a battery or a rectifier. To measure the current you will need an ammeter, and to record the voltage you will need a voltmeter.
To place the mold and anodes in the galvanic tank, it is necessary to provide suspensions. The form is suspended on a copper or brass wire and placed in a container at a distance of 15-20 mm from the anode.
The electrodes connected to the positive terminal of the current source (anode) are also suspended on copper or brass, while the wire hooks are not immersed in the electrolyte, otherwise deformation of the suspensions is possible due to corrosion of the hook. The form is connected to the negative terminal of the current source.
Copper plates with a thickness of 3 mm or more are used as anodes. of sufficient size. The surface area of the anodes must exceed the surface area of the mold.
To control the temperature of the electrolyte, you can use a regular mercury thermometer.
Preparation of electrolyte for electroplating
copper sulfate in solution – 150-180 g/l. Copper sulfate powder is dissolved in hot water and, after cooling and filtering, sulfuric acid is carefully poured into it in small portions at a rate of 30-35 g/l.
If the content of copper sulfate in the solution is exceeded, copper sulfate begins to crystallize on the walls of the galvanic container and on the anode; in this case, it is necessary to analyze the electrolyte (see.
“Analysis and adjustment of copper plating electrolyte”) and, according to the results, add water or acid.
An excess of sulfuric acid in the electrolyte can lead to copper deposits that are brittle and fragile. Lack of acid causes the deposition of a loose and porous layer.
To improve the quality of the resulting copper deposits, experts advise adding alcohol to the electrolyte in an amount of 8-10 g/l. Alcohol in a small amount improves the structure of the coating, making the copper finely crystalline, harder and more elastic.
The quality of the electrolyte and the resulting copper deposit may be negatively affected by the possible presence of organic impurities in the solution. To eliminate them, add 2-3 g/l of potassium permanganate or the same amount of crushed activated carbon to the heated solution. After cooling to 18-200C and filtering, the solution can be used.
When used intensively, the electrolyte must be filtered to remove sludge - powdered copper, graphite and dust.
Sludge gradually accumulates in the solution, settles on the bottom and walls of the container, fine particles form a suspension that can contaminate the resulting copper deposits.
The amount of sludge is influenced by the quality of copper used in the manufacture of the anodes, as well as the increased current density in the process.
The article Analysis and adjustment of copper plating electrolyte discusses the method for determining the content of copper sulfate and sulfuric acid in the electrolyte solution, and also provides a calculation of the number of components.
Electroforming process
The temperature of the electrolyte during the galvanic deposition of copper is 18-200C and can increase to 280C due to the release of heat during the electrolysis process.
The process begins at a minimum current density, which is maintained until a metal layer is formed on the surface. The operating current density is set only after the metal layer has covered the connected conductors.
The maximum current density in the process depends on the thickness of the conductors, which in turn depends on the size of the future composition and the mold material.
In any case, the higher the current density, the more intense the metallization process.
It is better to understand the features of the process using specific examples of using the electroplating method at home.
Copying bas-reliefs, embossings, medals, clay and plasticine products
To make copies of such objects, plaster molds are used. Making a plaster mold is simple:
- gypsum is diluted in water until a creamy mass is obtained;
- the surface of the object to be copied is lubricated with a solution of paraffin in kerosene (for easy dismantling of the mold after the plaster has hardened);
- apply a thin layer of gypsum with a brush to the surface of the product (to prevent the formation of pores);
- a side is installed around the mold (to prevent the plaster from spreading);
- fill the surface of the product with gypsum (gypsum sets quickly, so they do it quickly);
- remove the mold after the plaster has dried;
- I connect conductors to the mold and install it in a galvanic bath (see What is electroplating. Connecting molds to a current source).
Metallization of lace
Metallization of lace compositions is an interesting technique of electroforming, in which woven or knitted lace, tulle lace and other compositions made from threads are covered with a layer of metal. Such products can serve as decorative elements of various artistic compositions, or be used directly for the manufacture of such compositions.
Copper quickly darkens in air; therefore, as a rule, lace compositions metallized with copper are additionally coated with a thin layer of precious metal using the electroplating method. Electroplating silvering or gilding is carried out in the usual way.
The production of a metal lace composition occurs as follows:
- the lace is stretched and attached to a frame made of wire (insulated) or wood;
- impregnate the material with a wax composition for electroforming;
- place the material between two sheets of paper and iron it to remove excess wax;
- an electrically conductive layer is applied - finely dispersed graphite or a conductive composition;
- connect thin copper conductors and install the frame in a galvanic container.
- the material coated with a layer of copper is removed from the electrolyte, removed from the frame and given the required shape or mounted on the product to be decorated.
- The increased layer of copper is coated with a layer of silver (galvanic silvering) or oxidized (see the article Silver plating at home).
Making metal leaves or herbariums
Metallization of tree leaves does not differ from other electroforming techniques except for the method of obtaining the form. An imprint from the sheet can be obtained on a wax composition.
The heated wax is poured into a pre-made shell with low sides and allowed to cool until the surface of the wax composition hardens but remains elastic.
The sheet is placed on the surface of the wax and pressed against the glass. After which the glass is removed along with the sheet. A clear imprint of the leaf should remain on the surface of the wax composition.
In a similar way, an imprint of the reverse side of the sheet is made.
After the wax has completely cooled, finely dispersed graphite is carefully applied with a soft brush, copper conductors are connected, weights are installed and the mold is placed in a galvanic container.
Further work with the metal sheet print is a creative process. The result should be a metal sheet that follows the shape of the sample and exactly copies its surface.
Copper coating of wood products
Small wooden decorative elements are coated with a layer of metal to give them the appearance of cast products.
Before applying a layer of conductive substance (graphite), wooden products are impregnated (boiled) in a wax mixture, paraffin, ceresin or ozokerite.
Otherwise, due to its hygroscopicity, the wood will absorb electrolyte. Then graphite is applied to the products, conductors and weights are connected and dipped into the electrolyte.
The process is no different from the metallization of gypsum compositions.
Metallization of bird feathers
Bird feathers are dipped into a molten wax composition, paraffin, ceresin or ozokerite, then graphitized, a thin copper wire is attached, a weight is suspended and lowered into an electrolyte.
Metallization of fruits, plants and flowers
To plate plants and fruits with metal, you will need to first coat them with a thin layer of silver. To do this, plants are dried, treated with alcohol or a solution of sodium, barium or calcium chloride. Then prepare solutions:
- Sodium hydroxide 4 g per 100 ml of distilled water.
- Silver nitrate 4 g per 100 ml of water.
- Ammonia 7 g per 100 ml of water.
- Sugar 2.5 g per 85 ml of water.
Then the solutions are poured into a container and the plant is immersed in the solution. The surface is coated with a thin layer of silver (chemical silvering). The plant or fruit is then subjected to galvanic copper plating.
The methods of metallization of various products and forms described in the article are an example of the application of electroplating methods at home and in an art workshop.
The processes of galvanic copper plating are described in detail in the articles: Electroplating at home, Copper plating and can be applied to products made from various materials, including dielectrics, with a conductive layer applied.
Alkaline (alkaline) battery
The alkaline zinc-manganese dioxide or "alkaline" voltaic cell battery provides much higher energy density and therefore capacity than the carbon-zinc or manganese-zinc-chloride type. It is also capable of higher discharge current.
Manganese dioxide (MnO2) and carbon form the positive electrode, while zinc is in powder form as the negative electrode (anode), which actually mixes to form a gel/paste with potassium hydroxide (KOH) with zinc powder as the electrolyte. Although the alkaline battery is more expensive and somewhat heavier, it is superior to carbon-zinc or chloride types. Additionally, alkaline batteries are known to be durable due to their ability to avoid the corrosive effects of acidic ammonium ion on zinc. Alkaline voltaic battery cells are particularly suitable for applications that involve relatively high levels of discharge current.
Method one: lemon battery
This homemade battery will use a citric acid-based electrolyte found in lemon pulp. For electrodes we will take copper and iron wires, nails or pins. The copper electrode will be positive, and the iron electrode will be negative.
The lemon needs to be cut crosswise into two parts. For greater stability, the halves are placed in small containers (glasses or shot glasses). It is necessary to connect the wires to the electrodes and immerse them in the lemon at a distance of 0.5 - 1 cm.
Now you need to take a multimeter and measure the voltage on the resulting galvanic element. If this is not enough, then you will also need to make several identical lemon batteries with your own hands and connect them in series using the same wires.
Homemade battery from improvised means
A battery or galvanic cell is a chemical source of electric current. All batteries sold in stores essentially have the same design. They use two electrodes of different compositions. The main element for the negative terminal (anode) of salt and alkaline batteries is zinc, and for their positive terminal (cathode) is manganese. The cathode of lithium batteries is made from lithium, and a variety of materials are used for the anode.
The electrolyte is located between the electrodes of the batteries. Its composition is different: for salt batteries, which have the lowest resource, ammonium chloride is used. Alkaline batteries use potassium hydroxide, while lithium batteries use an organic electrolyte.
https://youtube.com/watch?v=chfVwKF5R1A
When the electrolyte interacts with the anode, an excess of electrons is formed near it, creating a potential difference between the electrodes. When the electrical circuit is closed, the number of electrons is constantly replenished due to a chemical reaction, and the battery maintains the flow of current through the load. In this case, the anode material gradually corrodes and breaks down. When it is completely used up, the battery life is exhausted.
Despite the fact that the composition of the batteries is balanced by manufacturers to ensure long and stable operation, you can make the battery yourself. Let's look at several ways you can make a battery with your own hands.
Making a battery
Now let’s solve the question of how to make a battery with your own hands at home. More precisely, not yet a battery, but a single battery. Let's start with the most interesting design in terms of operating principles - a gas battery. To assemble such a current source, we will need:
- two carbon rods;
- Activated carbon;
- opaque container;
- cotton fabric;
- needle and thread;
- salt.
Carbon rods can be obtained from salt batteries, and those that have expired will also be suitable. Activated carbon is sold in pharmacies in tablets. A plastic cup painted or covered with opaque paper is suitable as an opaque container.
Unlike galvanic cells, both electrodes in gas batteries are made of the same materials and have the same design. But let's get down to business. Let's start with the extraction of electrodes. It is better to take D-size salt batteries - they have larger cores. It will not be difficult to get graphite out of them. We flare the upper side of the upper part of the battery, pry up the metal cover with an awl and remove it. There is a plastic gasket under the cover. We remove it with an awl too.
We see a graphite rod stuck into a black dense mass. We pick out the mass with an awl and remove the rod.
Now we take two mined graphite rods, attach wires to their ends in one way or another - these will be current leads. We sew two bags from cotton fabric of such a size that they contain approximately 80–100 grams of powdered activated carbon.
It's coal's turn. It is sold in tablet form, but we need it in powder. We crush the tablets in a mortar and mortar for a long time until we get very fine dust. We pour it into bags, insert coal rods, tamp it down, add more if necessary. We sew up our bags as carefully as possible and wrap them tightly with thread. The better we wrap it, the better the contact between the coal and the graphite rod will be.
We install the electrodes in a cup and separate them with some kind of separator. In the photo below, ordinary wood chips are used as a separator, but it is better to make something more ion-permeable. For example, thick batting.
The electrodes are in place, the electrolyte is ready
Fill in the electrolyte (100 g of salt per 1 liter of water), but not to the top - the contacts should remain dry. The battery is ready, but it still needs to be charged. Since we have the same electrodes, we arbitrarily choose which one will be positive and which negative. You can start charging. To do this, you will need a DC source with a voltage of 4.5–5 V. For example, a charger from any five-volt gadget will do. We connect it to the terminals and start charging.
Healthy! Before charging, it is better to wait 10–15 minutes so that the coal in the bags is properly saturated.
During the charging process, electrolysis of the electrolyte (sodium chloride + water) begins. As a result of electrolysis, hydrogen accumulates on one of the electrodes, and chlorine on the other. It is stored in the pores of activated carbon in its pure form and does not interact with anything. As soon as the “containers” are filled, a violent release of gases will begin (the battery will “boil”, since hydrogen and chlorine have nowhere else to accumulate). By that time, the voltage at the battery terminals will be 2.2–2.4 V.
With these dimensions, our rechargeable battery will have a capacity of approximately 1 Ah, and the short circuit current will be approximately 300 mA. As the discharge progresses, the voltage will decrease until it drops to zero - the device is not afraid of a complete discharge. And if desired, you can even reverse the polarity by charging it the other way around. While we discharge the battery, gases will leave the electrodes, reducing salt and water. What are the disadvantages of gas batteries? The main one is high self-discharge. Even if the load is completely turned off, the electrodes will lose gas, and literally after a couple of days the source will have to be charged again.
Expert opinion
Alexey Bartosh
Specialist in repair and maintenance of electrical equipment and industrial electronics.
Ask a Question
Important! To reduce self-discharge, it is necessary to completely protect the electrodes from light - take an opaque body and make a light-proof cover.
Now about lead batteries. Of course, good performance can be achieved from a battery of this type (as well as from an alkaline one). But the process of manufacturing a high-capacity lead battery is so expensive and labor-intensive that it makes no sense to manufacture a current source of this type. Therefore, we will not bother with making a lead-acid battery, but will simply watch an entertaining video. And if there is a desire, everyone can repeat this experiment on their own.
Homemade lead-copper battery
Everything seems simple, but, as we see, the results are disappointing. But there is no point in casting the plates yourself, applying lead oxide and dioxide on them, as noted above.
Method four: battery in a beer can
The anode of the battery is the aluminum body of a beer can. The cathode is a graphite rod.
- a piece of foam more than 1 cm thick;
- coal chips or dust (you can use what’s left from the fire);
- water and regular table salt;
- wax or paraffin (candles can be used).
You need to cut off the top part of the can. Then make a circle of foam plastic the size of the bottom of the jar and insert it inside, having previously made a hole in the middle for the graphite rod. The rod itself is inserted into the jar strictly in the center, the cavity between it and the walls is filled with coal chips. Then an aqueous solution of salt is prepared (3 tablespoons per 500 ml of water) and poured into a jar. To prevent the solution from spilling out, the edges of the jar are filled with wax or paraffin.
You can use clothespins to connect the wires to the graphite rods.
Area of use
Electrochemistry has many important applications, especially in industry. Its processes are used to make electric batteries. They have many uses including:
- A fuel cell converts chemical potential energy obtained from the oxidation of fuels, such as gas, hydrogen, hydrocarbons, and alcohols, into electrical energy.
- Various types of piezo lighters for gas.
- Electrical appliances such as mobile phones.
- Digital cameras are lithium.
- Hearing aids (silver oxide).
- Electronic watches (mercury/silver oxide).
- Military power sources (thermal).
- Batteries A, AA, AAA, D, C and others.
Using chemical reactions to produce electricity is currently a priority for many researchers. The ability to adequately utilize chemical reactions as a source of energy will greatly help solve environmental pollution problems.
Method three: copper coins
The ingredients for making such a battery yourself are:
- copper coins;
- aluminium foil;
- thick cardboard;
- table vinegar;
- wires.
It is not difficult to guess that the electrodes will be copper and aluminum, and an aqueous solution of acetic acid is used as the electrolyte.
Coins first need to be cleaned of oxides. To do this, you will need to briefly dip them in vinegar. Then we make circles from cardboard and foil according to the size of the coins, using one of them as a template. We cut out the mugs with scissors, put the cardboard ones in vinegar for a while: they should be saturated with electrolyte.
Then we lay out a column of ingredients: first a coin, then a cardboard circle, a foil circle, a coin again, and so on until the material runs out. The final element should again be a copper coin. You can solder wires to the outer coins in advance. If you don’t want to solder, then the wires are attached to them, and the entire structure is tightly wrapped with tape.
During the operation of this DIY battery, the coins will become completely unusable, so you should not use numismatic material that is of cultural and material value.
VOLTAGE CELLS: HOMEMADE BATTERY
Each of us is familiar with chemical current sources of various types and forms. But as often happens, we rarely think about how this completely familiar and ordinary object works. Meanwhile, the emergence of the first chemical sources of current marked the beginning of the transformation of electricity from a laboratory curiosity into our everyday assistant.
Another Italian scientist Alessandro Volta was able to give the correct explanation for this phenomenon. He found that this phenomenon was associated with the presence of two dissimilar metals in contact with the electrolyte, which was the blood of the frog, and the paw itself played only the role of a sensitive indicator of electric current. Based on his studies of Volta in 1799. created the first chemical current source. In this device, Volta used copper and zinc electrodes immersed in a sulfuric acid solution.
Zinc reacts violently with acids. It is not zinc atoms that pass into the solution, but positive ions, so that an excess of electrons remains in the electrode, therefore, the zinc plate is negatively charged. In general, most metals become negatively charged when immersed in an electrolyte; a similar process occurs on the surface of a copper plate. But the excess of negative charges on the copper electrode is much less, which means that its potential is higher relative to the zinc electrode. If you connect copper and zinc plates with an external conductor, then electrons will begin to move from the zinc plate to the copper one, i.e. electric current will flow in the circuit.
Often the voltage provided by one galvanic cell is not enough. Then they can be connected in series into batteries.
In general, making a chemical current source is not at all difficult: you need to place two plates of different metals in an electrolyte. Such galvanic cells arise spontaneously. For example, if rain wets a roof covered with galvanized iron, there are probably scratches on the iron, so that both iron and zinc come into contact with water, which acts as an electrolyte. The zinc in such a pair will begin to actively degrade, but the iron will not be affected until all the zinc is destroyed. This is why iron is coated with a layer of zinc.
Galvanic corrosion can be most clearly observed by the example of contacts of iron with zinc and copper in a salt solution. Iron clips were placed on zinc and copper plates and immersed in a salt solution.
A day later, the paperclip connected to the copper plate became covered with rust. While the paper clip, which was in contact with zinc, was not damaged at all.
Scientists have compiled an electrochemical series of metal voltages. The farther apart the metals are in this row, the higher the voltage produced by the galvanic cell composed of these metals. Thus, a gold-lithium pair can theoretically produce an electromotive force (EMF) of 4.72 V. But such a pair will not be able to work in an aqueous environment - lithium is an alkali metal that easily reacts with water, and gold is too expensive for such an application.
In practice, the Volta element has a number of serious disadvantages.
- Firstly, its electrolyte is a very caustic liquid - a solution of sulfuric acid. Liquid electrolyte is always inconvenient or even dangerous. It may splash or spill if the housing is damaged.
- Secondly, hydrogen will be released at the copper electrode of such an element. This phenomenon is called polarization. In many properties, hydrogen is very close to metals, so its bubbles will create an additional emf of polarization, tending to cause a current in the opposite direction. In addition, gas bubbles do not allow electric current to pass through, which also leads to a weakening of the current. Therefore, you have to periodically shake the vessel, removing bubbles mechanically, or introducing special depolarizers into the electrolyte.
- Thirdly, during the operation of the Volta galvanic cell, the zinc electrode gradually dissolves. Theoretically, when the galvanic cell is not used, the destruction of the zinc electrode should stop, but since zinc almost always contains impurities of other metals, when they come into contact with the electrolyte, they act as a second electrode, forming a short-circuited element, which leads to galvanic corrosion of the zinc electrode. In order to eliminate this drawback, it is necessary to use ultra-pure zinc or design to provide for the possibility of removing the zinc electrode from the electrolyte. So when the battery is not in use, the electrolyte should be drained from it.
But for demonstration purposes, all these disadvantages can be neglected if the sulfuric acid is replaced with a safer electrolyte.
How often there are situations when, on a hike, at the dacha, or somewhere else, we need to recharge the phone, or use a little light. Most often on a hike, when necessary save batteries, you need to call or do something else. So, let's
let's make a battery
from what we have on hand!
1. Battery from saline solution
To make a galvanic cell, we need:
1) A large vessel (a bucket, maybe even a holey one, or something like that, you can even use plastic bags) 2) A zinc and copper plate. If there are no plates, then you can simply use zinc and copper wire, but the plates have a larger area and produce more current. 3) Earth. Yes, you can just dig up some soil. 4) Saline solution. I won’t give any exact recommendations here. Half a pack of salt is enough for a bucket of water.
It’s simple - we fill it with soil, stick in the electrodes, water it, and at the ends of the electrodes you will see a voltage of about 0.5-1V. Of course, not much, but what’s stopping you from making a battery of such elements? Enough to charge a mobile phone. Pour it in, pour it in and go about your business!
A good option for a homemade element is an air-aluminum one. To do this, you need to take aluminum cathode foil, soak a napkin with salt (or sea water), I also tried using acidic flux, a pile of carbon powder as an anode, I took toner from laser printer cartridges. The voltage is 0.5-1.0V at a current of 10mA
2. Battery made from fruits and vegetables
To make a galvanic cell we need: two electrodes, an oxidizing agent, a reducing agent and an electrolyte. Let's take three plates: copper, iron and magnesium - they will serve as electrodes. To measure voltage, we need a voltmeter; a digital (or analog) tester is quite suitable for these purposes. And as a “glass” with electrolyte we use a large and beautiful... orange. Fruit and vegetable juice contains dissolved electrolytes - salts and organic acids. Their concentration is not very high, but that suits us quite well.
So, let's put an orange on the table and stick our three electrodes (copper, iron and magnesium) into it. Pre-attach a wire to each of the electrodes (it’s convenient to use alligator clips for this). Now connect the tester contacts to the copper and iron electrode. The device will show a voltage of about 0.4-0.5 V. Disconnect the contact from the iron electrode and connect it to the magnesium one. Between the copper and magnesium electrodes there will be a potential difference of about 1.4-1.5 V - approximately the same as that of a finger-type battery. And finally, the iron-magnesium galvanic cell will give a voltage of about 0.8-0.9 V. If you swap the contacts, the sign of the device will change (“+” to “-” or vice versa). In other words, current will flow through the voltmeter in the opposite direction.
Instead of an orange, you can use grapefruit, apple, lemon, onion, potato and many other fruits and vegetables. It is curious that batteries made from orange, apple, grapefruit and onion gave fairly close voltage values - the difference did not exceed 0.1 V. The reducing agent in our case is iron or magnesium, the oxidizing agent is hydrogen ions and oxygen (which are contained in the juice). Note that the iron in a copper-iron cell is negatively charged, while the iron in an iron-magnesium cell is positively charged. If you do not have magnesium, the experiment can be carried out with two electrodes - copper and iron. Instead of iron, you can take zinc or a piece of galvanized sheet. A zinc electrode should give a larger potential difference with copper and a smaller one with magnesium.
In the case of citrus fruits, the experiment looks especially beautiful if you cut the fruit crosswise so that the “slices” are visible and insert electrodes into them (usually this is how a lemon is cut). If the fruit is cut lengthwise, it will not look so impressive.
The figures given should not be taken as absolute. The voltage of our battery depends on the concentration of hydrogen ions (as well as other ions) in the juice of fruits and vegetables, the rate of oxygen diffusion, the condition of the surface of the electrodes and other factors. The voltage of the battery you make may differ significantly from what was observed in this experiment. You can connect several fruit batteries in series - this will increase the voltage in proportion to the number of fruits taken.
The same materials are suitable for a potato battery, but it produces less voltage, so it is recommended to add a little salt inside the potato, the effect will be much greater.
3. Coffee battery (Nespresso battery)
In an effort to show the world the importance of collecting and recycling valuable aluminum materials, Vienna-based designers at Mischer' Traxler have developed batteries from 700 used aluminum cans and coffee grounds to power a quartz watch.
The design developed was called the “Nespresso Battery”, the installation is made from old aluminum cans, coffee grounds, strips of copper and salt water. In the photo below:
- a clock as a testing device - salt - ground coffee - wires - copper plates - aluminum plates - glass - plastic bottle separator
In a glass we put a copper plate (textolite, coin, thick wire) and aluminum slices (from beer cans). To prevent copper and aluminum from coming into contact, we place a separator between them made of any dielectric (plastic from a bottle, coffee grounds), and it should not interfere with the free flow of water. We connect wires to the plates, one to copper and one to aluminum. Now take water and add a few tablespoons of salt there, mix them until the salt is completely dissolved. Pour this solution into a glass. The battery is all done.
Each battery produces enough energy for an electromechanical quartz watch. And 17 batteries are enough to operate a small radio. The device is one of three winners in a competition entitled "Sustainability by Design".
The coffee grounds here are purely for decoration, and so that you can give it a nice name. And so its function can be used to separate conductors, you can completely abandon coffee grounds.
4. Baghdad battery (Parthian battery)
A small Parthian vessel was found at Khuzhut Rabu, in the vicinity of modern Baghdad (now Iraq), once part of the western territories of Greater Iran. In June 1936, a new railway was being laid near Baghdad - and workers discovered an ancient burial place. Subsequent excavations revealed that it belongs to the Parthian period (c. 250 BC - 250 AD).
One of the finds was a clay vessel with an asphalt “stopper”. An iron rod passed through the “plug”. Inside the vessel, the rod was lowered into a copper cylinder. This vessel was first described by the German archaeologist Wilhelm Koenig in 1938 - he considered it very similar to an electric battery, and published an article on this topic in 1940.
Using a similar principle, you can assemble your own battery. We take a “vessel” that can be made from: clay, plasticine, a bottle, a jar, a glass, insert into it a copper plate twisted into a cylinder, and insert a nickel-plated nail into this cylinder. These plate and nail are electrodes, they should stick out a little from the can. To secure them in the body of the “vessel” you can use: epoxy glue, plasticine, window putty, etc. Now we need to make an electrolyte. It can be alkaline or acidic. For alkali, you need to make a concentrated solution of: water + salt or water + soda. For acidic, diluted acetic or oxalic acid in water is suitable, or you can use citrus juice.
Pour the electrolyte inside the jar and carefully seal the “vessel”. The Baghdad battery is ready.
When such a model is filled with electrolyte, it can produce voltage. In general, depending on the type of electrolyte, the voltage supplied by the “battery” varies from 0.5 to 2 volts.
Unfortunately, due to the destruction of many Iranian literary sources and libraries during enemy invasions of Iran over the centuries, there are no written records of what exactly such vessels were used for. Everything we know about them today is just guesswork.
5. Solar battery
Having read on the endless expanses of the Internet about homemade solar cells, I decided to conduct my own “experiments” in this area. I will tell you about the easiest way to make solar panels with your own hands.
To begin with, I decided to decide on the element base. For a solar cell we need PN junctions. They are found in diodes and transistors. It was decided to choose KT801 silicon transistors. They were produced in a metal case and therefore they can be opened without damaging the crystal. It is enough to press the lid with pliers and it will break off.
Now let's look at the parameters. In average daylight, each of our transistors produces 0.53V (Base is plus, and Collector and Emitter are minuses). And then there is one nuance. Transistors from 1972 have a large white crystal, and produce about 1.1mA. Transistors from 1973 to 1980 The releases have a large crystal with a green coating, and produce about 0.9mA. Transistors released later have small crystals and produce only 0.13mA.
For the experiment, I used a battery of two parallel chains of 4 transistors. Under load it produced about 1.8V, 2-2.5mA. These are rather modest parameters, but as they say, “for free”. You can power a Chinese wristwatch with such a battery, or charge the battery and power an LED, bug, etc.
For ease of mounting and measurements, you can mount the transistors on a printed circuit board as in the figure below. My device is wall-mounted, as this speeds up assembly.
6. Coin-energy battery
It seems that the design is standard, zinc-copper contacts and salted water, but the design of the battery itself is interesting.
We will need:
- ice tray - copper/copper alloy coins - nickel/aluminum bronze/zinc coins - paper clips - salt - water - LED (for checking)
To get a battery, you need to connect coins into electrodes and fill them with electrolyte. In each cell of the tray you need to place two coins from different alloys, for example copper and nickel. Next, we connect all the cells in series using a paper clip. Pressing a copper coin to one side of the wall and a nickel coin to the other, we secure them with a paper clip. After this, you need to fill each tray with electrolyte: salt + water. Pay attention to the ends of the tray, since the cells are in two rows, on one side we need to connect them, and on the other they should remain unconnected. Now we check the performance of the battery using a diode or a multimeter; to do this, we close two unconnected cells with it.
One cell produces electricity with a voltage of 0.5 V, and those connected into one battery produce 2 V and 110 mA. Therefore, it is desirable to have one electrolyte for all cells, and not heterogeneous ones.
Peculiarities:
1. The cell should be completely filled with electrolyte, but contact should only be with a coin, not a paper clip. 2. One of the pairs of cells should not be shorted to each other. 3. Zinc coins are used as positive electrodes, and copper coins are used as negative electrodes. 4. Coins must be made of different metals/alloys (copper and nickel), it is also desirable that they do not contain the same impurities in the alloys.
7. Homemade battery
Now we will make a fairly simple device, or rather a power source - a homemade voltage battery. As is known, two different metals immersed in an electrolyte solution are capable of accumulating electric current. It was decided to use copper and aluminum foil as electrodes (in my opinion, they are the most affordable).
In addition to foil, we also need a sheet of paper, transparent tape and the vessel itself in which we will place the battery jar (it is very convenient to use a glass vessel made from naphthysine or valerian tablets).
Let's look at the photographs.
The foils are almost the same size, only the aluminum foil is a little longer, there is no reason for this, it is simply easier to apply solder to copper foil than to aluminum foil and the wire is not soldered to the foil, it is simply rolled into it and then clamped with pliers.
Next, both foils were wrapped in a sheet of paper. It is not permissible for metals to touch each other; a sheet of paper serves as a barrier between them. Then the foils need to be taken together and wrapped in a circle and wrapped with thread or transparent tape.
Then the completed package must be placed in a vessel. After this, take 50 ml of water and dilute 10 - 20 grams of salt into it. Mix the solution thoroughly and heat until all the salt has melted.
After melting the salt, pour the solution into a vessel where we have a ready-made blank for our homemade battery. After filling, wait a few minutes and measure the voltage on the battery wires. I forgot to specify the polarity of the battery, copper foil is a plus for power supply, aluminum foil is a minus. Measurements will show a voltage of the order of 0.5-0.7 volts. But the initial tension doesn't mean anything. We need to charge our battery. You can charge from any DC source with a voltage of 2.5-3 volts, charging lasts half an hour. After charging, we measure the voltage again, it increased to 1.3 volts and can reach up to 1.45 volts. The maximum current of such a homemade battery can reach up to 350 milliamps.
You can make several of these batteries and use them as a backup power source for, say, an LED panel or flashlight. To increase the power of the battery, you can use large foil, but of course such a homemade battery will not hold a charge for very long (the charge will run out within one week), another disadvantage is the short service life (no more than 3 months), since oxide forms on copper During the charge-discharge process, the aluminum foil begins to corrode and will gradually separate into small pieces, but I think for experiments it’s worth trying to assemble such a simple battery.
8. DC adapter
Having a little free time and desire, it’s easy to assemble an adapter from scrap materials to power various gadgets from an external power source.
What I liked about this article is the simplicity of this adapter. I will describe the manufacturing technology in more detail. I think it will be useful to someone else, especially since there is absolutely nothing complicated here. I didn’t even go anywhere to get the material. There was an old MTS card lying on the table. It was not in vain that he paid a hundred rubles. I tried it on, it’s exactly suitable for making a model of one battery for a camera. Cutting cardboard:
There are even very few scraps left.
The cardboard is what you need - hard, about 0.25 mm thick. I made markings and cut along the seams. The cardboard was not cut all the way through, but about a little more than half the thickness, to make bending and gluing easier. For contacts, I riveted 1.5 square millimeter copper wire. It turned out something like this.
This is what the contacts look like from the inside:
I soldered the wires and double-glued all the seams with Moment STOLYAR PVA glue. The seams are thin, so I had to smear them patiently, drop by drop, with the tip of a toothpick... Although, if you can’t wait, you can glue them together with tape. We connect to the “vampire” and work:
Connected it, everything worked.
So far, only one inconvenience has been discovered - the wire. He’s fat, reaching for the camera and the “vampire little one.” Therefore, I decided to attach to the camera the same battery as in the “vampire little one,” only with protection. By the way, it is not necessary to install batteries with protection here, because... The camera already has a built-in charge level meter and if the battery is low it simply will not turn on.
And don't forget to observe polarity!!!
>
Random site materials
How to properly sharpen a knife 10138
Lithium battery
The lithium manganese dioxide battery is a relatively recent development that takes advantage of the high electrode potential and energy density of lithium metal. It offers significantly greater energy density and capacity than alkaline and carbon at a relatively small increase in cost.
The lithium is in the form of a very thin foil and pressed inside a stainless steel can to form the negative electrode.
The positive electrode is manganese dioxide mixed with carbon to improve its conductivity, and the electrolyte is lithium perchlorate dissolved in propylene carbonate.
The rated terminal voltage of a lithium cell is 3.0V, which is twice that of alkaline and other voltaic cells. It also has a very low self-discharge rate, giving it a very long shelf life. The internal resistance is also quite low and remains so throughout its service life.
Lithium batteries perform well at low temperatures, even below -60°C, and advanced designs use them in communications satellites, spacecraft, military and medical applications. Medical applications that require long life for critical devices, such as artificial pacemakers and other implantable electronic medical devices, use specialized lithium-ion batteries that can last for many years.
Lithium voltaic batteries are suitable for less critical applications such as toys, watches and cameras. Although lithium batteries are more expensive, they provide longer life than alkaline batteries and reduce battery replacement to a minimum.
In practice, however, the terminal voltage decreases as the charge decreases. It is for this reason that, unlike secondary batteries, primary batteries typically do not receive a capacity specification in either amp-hours or milliamp-hours from most manufacturers; instead, only the maximum discharge current is usually specified. Lithium voltaic cells have significantly greater energy density and capacity than alkaline and other primary batteries; they provide higher (about twice) terminal voltage compared to other primary elements, and the terminal voltage remains almost constant throughout their service life.
Causes of coating defects
There are several defects:
- Uneven shine - too much current or low solution temperature.
- Lack of shine - insufficient amount of sulfuric acid or anhydride.
- The appearance of brown spots is a lack of sulfuric acid or an excess of chromic anhydride.
- Low coating strength - low current or high electrolyte temperature.
Types of galvanic cells
The most common are carbon-zinc elements. They use a passive carbon current collector in contact with the anode, which is manganese oxide (4). The electrolyte is ammonium chloride, used in paste form.
It does not spread, which is why the galvanic cell itself is called dry. Its feature is the ability to “recover” during operation, which has a positive effect on the duration of their operational period. Such galvanic cells have low cost, but low power. As the temperature drops, they reduce their efficiency, and as the temperature rises, the electrolyte gradually dries out.
Alkaline cells require the use of an alkali solution, so they have quite a few areas of application.
In lithium cells, the active metal acts as an anode, which has a positive effect on the service life. Lithium has a negative electrode potential, therefore, with small dimensions, such elements have a maximum rated voltage. Among the disadvantages of such systems is the high price. Opening lithium power sources is explosive.
Making a battery
When making a demonstration battery of galvanic cells, we will use a standard pair - copper and zinc. Copper foil can be found in some transformers. As a last resort, you can make a copper electrode from coiled bare copper wire. Zinc can be extracted from discharged salt batteries; as a rule, quite a lot of metallic zinc remains in them even when the element is unsuitable for further use. Instead of an acid solution, take a 10% solution of table salt. Plastic vitamin containers with a volume of approximately 50-100 ml were taken as a container for the electrolyte.
Screws are used as contacts, which simultaneously secure the electrodes to the cover. In this case, it is highly advisable to fasten the copper electrodes with a brass screw. The zinc plate can be fastened with a steel screw without any problems. For sealing, a suitable sized rubber plumbing gasket is placed under the nut.
A battery of three galvanic cells allows you to power the LED.
The voltage per battery cell is about 1 V.
The current supplied to the load is about 0.23 mA
This current is enough to light the LED. However, this glow can only be seen in a photograph if you shoot at high light sensitivity.
Such a battery can be used at school, for example, to perform laboratory work on determining the internal resistance of a current source.
Equipment for preparing electrolyte
To store chemical reagents and electrolytes, you need glass containers with ground-in lids.
To prepare electrolytes, the necessary substances must be measured to the nearest gram. For this you need appropriate scales. You can purchase them or make them yourself, using Soviet coins as weights, the denomination of which exactly corresponds to the mass.
Electroplating at home also means preparing an electrolyte from chemical reagents obtained through difficult means. Specialized companies selling such high-quality substances are not uncommon, but their activities are controlled, and working with them requires special permits, even for legal entities. Hazardous chemicals are not sold to private individuals.
Design and principle of operation of a galvanic cell
A metal immersed in an electrolyte solution is called an electrode.
Electrodes are a system of two conductive bodies - conductors of the first and second kind.
Conductors of the first kind include metals, alloys, oxides with metallic conductivity, as well as non-metallic materials, in particular graphite; charge carriers are electrons.
Conductors of the second type include melts and solutions of electrolytes; charge carriers are ions.
A device consisting of two electrodes is called a galvanic cell.
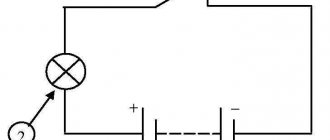
| Rice. 2. Scheme of a copper-zinc galvanic cell |
Let's consider a Jacobi-Daniel galvanic cell (the diagram is shown in Fig. 2). It consists of a zinc plate immersed in a zinc sulfate solution and a copper plate immersed in a copper sulfate solution. To prevent direct interaction between the oxidizing agent and the reducing agent, the electrodes are separated from each other by a porous partition.
In a galvanic cell, an electrode made of a more active metal, i.e. The metal located to the left in the voltage series is called the anode, and the electrode made of a less active metal is called the cathode.
An electric double layer appears on the surface of the zinc electrode (anode) and equilibrium is established:
Zn0 – 2ē ←→ Zn2+.
As a result of this process, the electrode potential of zinc arises.
A double electrical layer also appears on the surface of the copper electrode (cathode) and equilibrium is established:
Cu2+ + 2ē ←→ Cu0.
As a result, the copper electrode potential arises.
Since the potential of the zinc electrode has a more negative value than the potential of the copper electrode, when the external circuit is closed, i.e. When zinc is connected to copper with a metal conductor, electrons will flow from the zinc to the copper. As a result of this process, the equilibrium at the zinc electrode shifts to the right, so an additional amount of zinc ions will pass into the solution. At the same time, the equilibrium at the copper electrode will shift to the left and a discharge of copper ions will occur.
Thus, when the external circuit is closed, spontaneous processes of zinc dissolution on the zinc electrode and copper release on the copper electrode occur. These processes will continue until the potentials are equalized or all the zinc is dissolved or all the copper is deposited on the copper electrode.
So, during the operation of a Jacobi-Daniel galvanic cell, the following processes occur:
1. Anodic process, oxidation process:
Zn0 – 2ē → Zn2+.
2. Cathode process, reduction process:
Cu2+ + 2ē → Cu0.
3. Movement of electrons in an external circuit.
4. Movement of ions in solution: SO42– anions to the anode, Cu2+ cations to the cathode. The movement of ions in the solution closes the electrical circuit of the galvanic cell.
Summing up the electrode reactions, we get:
Zn + Cu2+ = Zn2+ + Cu.
As a result of the occurrence of this reaction in a galvanic cell, the movement of electrons in the external circuit and ions inside the element occurs, i.e. electricity. Therefore, the total chemical reaction occurring in a galvanic cell is called a current-generating reaction.
The electric current in a galvanic cell arises due to a redox reaction, which proceeds in such a way that the oxidation and reduction processes are spatially separated: the oxidation process occurs on the negative electrode (anode), and the reduction process occurs on the positive electrode (cathode).
A necessary condition for the operation of a galvanic cell is the potential difference of the electrodes. The maximum potential difference between the electrodes that can be obtained when operating a galvanic cell is called the electromotive force (EMF) of the cell. It is equal to the difference between the cathode potential and the anode potential of the element:
EMF = Ek – Ea. (1)
The EMF of an element is considered positive if the current-generating reaction in a given direction occurs spontaneously. A certain order in the recording of the element circuit corresponds to a positive EMF: the electrode written on the left must be negative. For example, the Jacobi-Daniel element circuit is written as:
Zn │ ZnSO4 ║ CuSO4 │ Cu .
Basic methods of chrome plating
- Chemical metallization process.
- Galvanization.
- Vacuum spraying.
- High temperature diffusion.
The last two methods are used only in industrial enterprises. These processes cannot be performed at home, since they require technically complex installations and increased energy costs. But chemical metallization and galvanization are exactly the same processes that can be carried out in a personal workshop. Let's take a closer look at how this is done.
In the process of this work, chemical reagents, a compressor and a spray gun are used. Almost the same operations are performed as when painting surfaces with acrylic varnish or enamel. When chrome plating in this way, not a protective polymer film is applied to parts and structures, but a mirror-like thin layer of metal.
1st method. The surface is coated with special chemicals. As a result of the chemical reaction, a precipitate is formed, which is a durable metal layer. The coating can be made not only from chrome, but also from silver.
2nd method. During the process of a reducing chemical reaction, a layer of chromium is formed from the salts. Prepare a set of acetic acid, chromyl chloride, chromium chloride, chromium acetate, sodium hyposophite, sodium hydroxide, chromium phosphate. It must be remembered that these substances are very toxic and hazardous to health.
And therefore, you should carefully study the chemistry textbook if you decide to carry out the chrome plating process using this method. But achieving high quality is very difficult, even with detailed instructions. Despite all the existing disadvantages, this method is most often used for chrome plating surfaces in home workshops.
It is very convenient to carry out the plating process using ready-made test kits of chemicals for chrome plating. They are offered by Fusion Technologies. The convenience of such kits is that the coating can be applied to any of the listed materials: metal, ceramics, wood, glass, plastic.
Receiving a defective coating should not frighten a novice electroplater. A poor-quality layer of chromium can be removed in a solution of hydrochloric acid (100-200 g/l). After this, the parts are washed in water, and the chrome plating process can be repeated.
Most often there are several main defects:
- Peeling of the chrome film. The main reason is poor adhesion due to insufficient degreasing. After removing the coating, the surface is cleaned and reactivated again.
- Chromium growths (dendrites) on sharp edges and corners. This defect indicates a high current density on sharp edges. If possible, it is better to round the edges or install screens in problem areas.
- Matte finish. To achieve shine, it is necessary to increase the temperature of the solution, reduce the current, or add chromic anhydride.
Galvanic cell at home
You can make a simple current source with your own hands. To do this we need the following equipment:
- Plastic cup.
- Electrolyte. You can use a saline solution, soda or citric acid diluted in water.
- Plates of two different metals. For example, aluminum and copper.
- Wires
Manufacturing process
Take a plastic cup and pour electrolyte into it. Do not fill the glass to the very brim. It’s better not to add 1-2 centimeters. Attach the conductors to the metal plates. Next, install copper and aluminum plates on the edges of our container. They should be parallel to each other. When everything is ready, you can measure the voltage with a voltmeter.
Connect the device and touch the probes to the contacts of our current source. Hold them and do not tear them off until the voltage appears on the display. Typically it is 0.5-0.7 volts. Such numbers are shown depending on the electrolyte. More precisely the substance used in its quality.
In this way, a homemade galvanic cell is made.
Galvanoplasty and galvanostegy
What is galvanoplasty? This is a method that is used to make exact copies of products, the copying method. It is used when it is necessary to make a copy of objects of the finest configuration - records, chips and circuits. Electroplating allows you to enhance the mechanical properties of one metal by applying a layer of another metal to it, for example, chrome and nickel plating of steel, nickel plating of copper, etc.
Galvanoplasty and galvanostegy are of a similar nature, differing only in the method of preparing the metal before processing. When performing electroplating, the metal surface must be as prepared as possible for adhesion to the applied metal. The electroplating method, on the contrary, involves the free separation of the applied metal.
Copper, nickel and silver are most often used for electroplating processes, and almost all types of metals are used in electroplating processes. Home electroplating is performed using the same equipment as other electroplating processes.
A large glass container is perfect for a galvanoplastic bath. Its dimensions depend on the size of the item being galvanized, since it should not be located too close to the anode plate.
Electroforming at home can be used to make copies of small-sized objects using molds pre-cast from low-melting metals.
Master class on galvanizing (1 video)
Items with galvanic coating (17 photos)
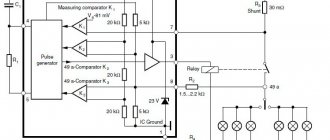
Galvanic cell diagram
Galvanic cell device
The simplest energy storage device consists of:
- Coal rod.
- Two dissimilar metals.
- Electrolyte.
- Resin or plastic.
- Isolator.
As can be seen from this diagram, the structure of the galvanic cell contains a negative and a positive electrode. They can be made of copper, zinc and other metals. They are named after the copper-zinc type. Sometimes they are called dry batteries.
The designation of a galvanic cell in the diagram is made in the form of two vertical straight lines close to each other at a short distance. One of which will be smaller. Along the edges near each such line there are signs indicating polarity. The long line is given a plus, and the short line a minus. Voltage may be located nearby. This means that the circuit in which the battery is used operates only on this voltage.
Operating principle of a galvanic cell
The operation of a galvanic cell is carried out by the movement of electrons from one metal contact to another. There is some kind of chemical transformation going on. Read more about the thermodynamics of a galvanic cell and the formation of galvanic electricity here.
Answers to frequently asked questions
| Galvanic | Explanation |
| Battery | An energy source that operates through processes occurring in a limited miniature space. In particular, energy appears when a chemical reaction occurs. |
| Volta element or Voltaic pillar | This is an energy element first created by a scientist named Volt. |
| Process | The interaction between chemical elements resulting in the formation of electric current. |
| Discharge | This is the completion of a chemical reaction. That is, there will be no interaction between substances. Galvanic discharge is available in the game Warframe. In fact, this is a modification that is in great short supply. It is used for edged weapons. Polarity V2. |
| Galvanic contact | This is the contact between the electrodes and the solution. |
| Effect | The appearance of a difference between two contacts made of 2 types of metals. The value depends on the temperature and chemistry of the conductors. Essentially this is Volta's first law. |
| Connection/Communication/Circuit | Combining 2 or more sections of an electrical circuit with a current source. |
| Galvanic charge | Filling the battery with energy. |
Galvanization is the occurrence of chemical processes using electric current. The reaction reduces the amount of dissolved metal cations to such an extent that they eventually form a single coating on the metal electrode. As a result, the item becomes more durable, small dents disappear and its appearance becomes more attractive.
General idea of electroplating
Galvanic coating can be either technological or decorative-protective. It is a thin surface layer of metal that has a good aesthetic appearance (gold, silver) or anti-corrosion properties (zinc, copper) on metal or plastic products.
In general terms, metal electroplating at home looks easy.
This is interesting: Features of welding aluminum with an inverter at home